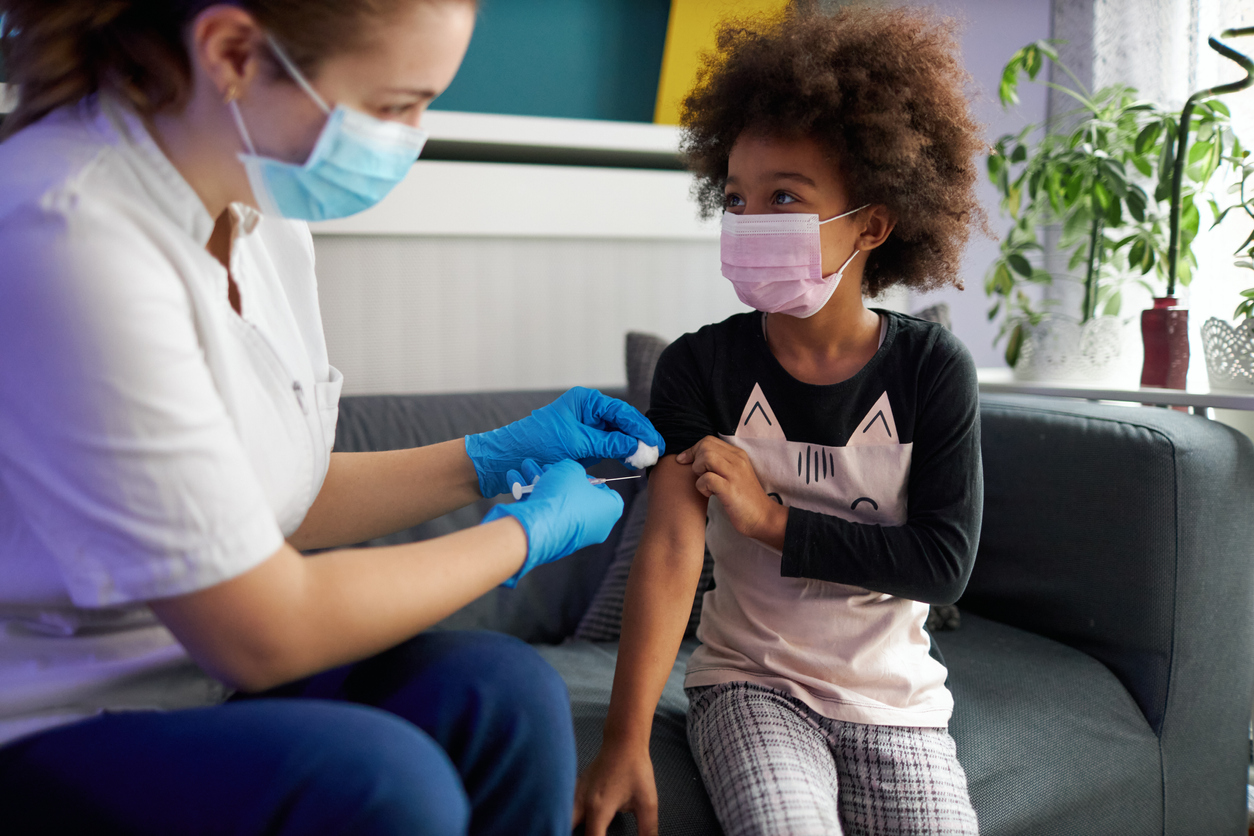
When the FDA met last week to discuss approving the vaccine, which was found to be 95 percent effective in a late-stage clinical trial, some doctors expressed concern that “there was not enough data from 16- and 17-year-olds to know whether the vaccine would help them,” The New York Times reports. “Several pediatricians on the committee expressed concern that Pfizer’s data so far with respect to the youngest participants was ’thin,’” The Times reports. According to Stat News, some panelists protested the inclusion of people younger than 18 at all, while others said the differences psychologically between a 16-year-old and an 18-year-old was minimal. If you want to learn more about the vaccination process, check out You Shouldn’t Do This Right After Getting a COVID Vaccine, Expert Warns. Only 163 people in Pfizer’s 44,000-person trial were as young as 16 or 17, NPR reports—and none were younger than 12. The FDA’s Peter Marks, MD, director of the Center for Biologics Evaluation and Research, noted that many 16- and 17-year-olds are working essential jobs at grocery stores and checkout counters. Some doctors also pointed out that people under 18 work in long-term care facilities, making them high risk for getting and potentially spreading the virus. And they also said that teenagers working these jobs may disproportionately be people of color, who have been particularly affected by COVID.ae0fcc31ae342fd3a1346ebb1f342fcb “We think the known and potential benefits outweigh the known and potential risks” of vaccinating this age group, Marks said, according to NPR. And for more on when the vaccine will actually turn our lives around again, check out Dr. Fauci Just Gave a New Timeline for Returning to Normal Post-Vaccine. After the FDA’s recommendation, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) met on Saturday voted 11 to 0 to recommend the vaccine for Americans 16 and older. And on Sunday, CDC Director Robert Redfield, MD, said, “Last night, I was proud to sign the Advisory Committee on Immunization Practices’ recommendation to use Pfizer’s COVID-19 vaccine in people 16 and older.” And for more on who the vaccine isn’t recommended for, check out These Are the Only People Who Shouldn’t Get the COVID Vaccine. Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases (NIAID), has been asked a lot about the COVID-19 vaccine and children. He told Meet the Press recently that “it’s going to be months” before medical professionals can say for certain that the vaccine is safe for kids. “Before you put [the vaccine] into the children, you’re going to want to make sure you have a degree of efficacy and safety that is established in an adult population, particularly an adult, normal population,” he explained. “We’re going to start the process very likely in January to get it to the children sooner rather than later.” And for more on signs you could be sick, check out This Is How to Tell If Your Cough Is COVID, Doctors Say.